Most nutrition studies focus on things like polyphenols, caffeine, or other chemicals released during brewing, but such research overlooks a unique aspect of tea: unlike most food and drink, tea leaves are not directly consumed, and the brewing process allows tea leaves to adsorb chemicals as well as release them—most notably heavy metal toxins like lead, arsenic, or cadmium. (Adsorption is when a substance adheres to the surface of something; absorption is when a material takes in a substance.)
Well, maybe I’ll start drinking tea.
So that’s why it’s so bitter. It removes the sweet, sweet lead from the water.
Seems to be an American problem.
I remember this making its rounds on HN lately. People are skeptical because of other numerous studies stating other findings that were contrary to this.
Can anyone get the official paper? https://pubs.acs.org/doi/10.1021/acsfoodscitech.4c01030 is the only real resource I can find and its a paywall.
Here you go:





Thank you!!
yeah there you go. no pdf bc it’s watermarked and i’m a bit too paranoid for that. supplementary info is free to read
ABSTRACT: The study of adsorbent materials rarely extends beyond engineered systems. This research explores metal adsorption resulting from the preparation of tea, which is the most widely consumed beverage globally. Tea is brewed from the processed leaves of the Camellia sinensis plant, a material understudied, relative to its outsized economic and cultural importance. The features that make tea leaves ideal for their intended purpose also help them function as an efficient sorbent. In this Letter, the properties of tea leaves relevant to the adsorption of metal content in drinking water are measured and used to estimate, for the first time, the adsorptive performance under a variety of conditions of tea preparation, with an eye toward public health benefits. We find that the preparation of black tea under typical conditions results in the removal of a meaningful fraction of lead from drinking water and that this value is highly dependent on steeping time. KEYWORDS: tea, heavy metals, lead, adsorption, water remediation, global health
- INTRODUCTION Tea is and has long been the most consumed beverage in the world, with its cultivation, trade, and consumption having played an immense role in human history.1,2 Today, tea is a vital economic good as a culturally and religiously important drink consumed more than beer, wine, milk, or coffee. As such, there is considerable interest in the health effects (both beneficial3,4 and detrimental5) of tea consumption. Some studies have specifically suggested that tea consumption can reduce metal intake, without proposing a definitive mechanism.6 While nutrition science often focuses on polyphenols, caffeine, and the other chemicals released by tea leaves,7 in this Letter we investigate a simple yet overlooked phenomenon. While the ingredients of many drinks are directly consumed, tea leaves are typically not, allowing them to function as an adsorbent for toxins such as heavy metals like lead, arsenic, and cadmium8−10 or be valorized into adsorbent materials for these applications.11−15 Previous research has suggested that tea leaves act as a carrier for heavy metals from contaminated soil.16 We argue that this is misguided, that the high specific surface area of tea leaves and the increased temperatures underlying tea preparation (the same features that allow tea leaves to quickly release flavor chemicals into water) lead to highly favorable partitioning of heavy metals from drinking water onto the surface of the tea leaves, lowering the heavy metal intake of tea drinkers. This mechanism may explain some of the health benefits of tea consumption in the literature and provides a link between these health benefits17,18 and the worldwide cultural heritage of tea consumption. We investigate the adsorptive properties of many varieties of the tea plant (Camellia sinensis) as well as related plants and tea bag materials and the effect of heating and other preparation conditions. In particular, we brew tea under typical conditions in water with increased levels of lead, a model contaminant, and then use thermodynamic and kinetic data from these adsorption experiments to estimate the projected lead remediation capabilities as a function of relevant parameters for tea preparation methods from around the world. The focus of this Letter is not prescriptive. We are not recommending the use of tea leaves as a sorbent or designing a new material for water remediation. Rather, we are quantifying the typical performance during the preparation of tea so to provide a potential explanation for those who study the health impacts of tea and a benchmark for the sorptive performance of other natural materials. By quantifying this effect, our work highlights the unrecognized potential of tea consumption to passively contribute to reduced heavy metal exposure in populations worldwide.
- MATERIALS AND METHODS 2.1. Tea Leaves. Tea leaves were purchased in bulk from a wholesaler (Davidson’s Organics) as well as in tea bags (Lipton Teas and Infusions). The following loose leaf teas (and herbal tea alternatives) were used for experiments: Yunnan Black, Imperial Green, White Peony, Quilan China Oolong, South African Rooibos, and Chamomile. Ground tea and tea bags from Lipton were also used. Cotton and cellulose tea bags were purchased from an Amazon retailer (Fenshine), and nylon tea bags were purchased from a separate Amazon retailer (Lucklovely). 2.2. Adsorption Testing. Stock solutions for each of the heavy metals tested were prepared from metal salts: lead(II) nitrate, sodium dichromate (hexavalent chromium), copper(II) chloride, zinc(II) acetate, and cadmium(II) nitrate. Adsorption trials at equilibrium were carried out over 24 h batch adsorption trials, with adsorbents added to 40 mL of metal-containing dilutions (Pb unless otherwise specified) of deionized water in plastic Falcon tubes. Tea leaves were removed from solution after the designated time by passing the liquid through a cellulose filter into a separate tube. Kinetics adsorption trials were performed using a larger volume of Pb-containing water with 5 mL aliquots removed at time intervals from the start. Negative controls without adsorbents added were performed for each experiment. To determine the elemental concentrations of Pb and other heavy metals, inductively coupled plasma optical emission spectroscopy (ICP-OES) and mass spectrometry (ICP-MS) were utilized. ICP-OES, performed on a Thermo iCap7600 instrument, has a detection threshold of 10 ppb and was used for experiments with higher concentrations, whereas ICP-MS, performed on a Thermo iCapQ instrument, was used for experiments near the detection limit of the ICP-OES or where the range of concentrations was below 1 ppm. To analyze the amount of metals adsorbed, concentrations before and after adsorption trials were compared via ICP analysis. Adsorbed amounts have been reported in milligrams of ion adsorbed per gram of adsorbent. Coefficients of determination are reported in the Supporting Information, when possible; for all other cases, error bars are reported directly on the graphs. 2.3. Modeling. Empirical thermodynamic data, analyzed using the Langmuir model, and kinetics data, fitted with a pseudo-second-order adsorption model, were utilized in a combined mathematical model in MATLAB to estimate metal remediation under a variety of conditions and for different methods of tea preparation.
- RESULTS AND DISCUSSION 3.1. Adsorptive Properties of Tea Leaves. There were two major goals for our experimentation: to understand whether the adsorptive performance during tea preparation is sufficiently significant to have a measurable effect on metal content and to see how sensitive this performance is to the conditions of tea preparation such as tea leaf varieties and processing, temperature, pH, and the concentration of the contaminating metal ion. The conditions chosen for experimentation and for modeling were selected not to optimize the adsorptive performance but rather because they were the conditions most relevant to understanding the typical performance during tea preparation. For example, black tea was selected for further study due to it being by far the most widely consumed variety of tea globally. Deionized water was selected for the majority of experimentation to minimize the confounding effects of competitive adsorption, although tap water was also tested to confirm that similar levels of adsorption occur even in the presence of competing ions. First, to measure the thermodynamic properties of the tea leaves at equilibrium across a range of concentrations, batch adsorption experiments were carried out over 24 h for three varieties of black tea, one variety of green tea, and cellulose tea bag material. These data were then fitted to Langmuir adsorption isotherms (shown in Figure 1a). The Langmuir parameters for the three black teas were later averaged to obtain an estimate of the thermodynamic properties of black tea leaves. The version of the Langmuir model used is shown in eq 1. The quantity adsorbed at equilibrium, qe, along with the concentration at equilibrium, Ce, is used to determine the two Langmuir fitting parameters, KL and qa, which represent the binding affinity of the adsorbent and the asymptotic limit of the amount of metal adsorbed, respectively. The cellulose tea bag was found to have a higher binding affinity but a lower asymptotic limit compared with those of the tea itself. The different tea leaves had similar values for both Langmuir parameters, with the Lipton tea�which was finely ground, packaged tea�having slightly increased properties compared to those of the whole tea leaves. The higher values for the Lipton tea line up with subsequent experimentation on the effect of fineness (see Figure 1d).= + q_e = (q_a K_L C_e)/(1+K_L C_e) (1) The kinetic properties of the adsorption process during tea preparation were probed at both room temperature and 85 °C, just below the boiling point of water. This temperature was selected because black teas are traditionally brewed around 90 °C and green and herbal teas at 80 °C, so the difference was split to maintain consistency across experiments. Moreover, this temperature ensures that variations in hot plate temperature will not cause the water to boil during adsorption experimentation carried out over multiple hours. The adsorption trials at room temperature were performed in still water, as well as in a beaker with a stir bar. Aliquots were removed from the tea as it “brewed” at time increments over the course of 4 h, with each sample tested for its Pb concentration.
continuing…
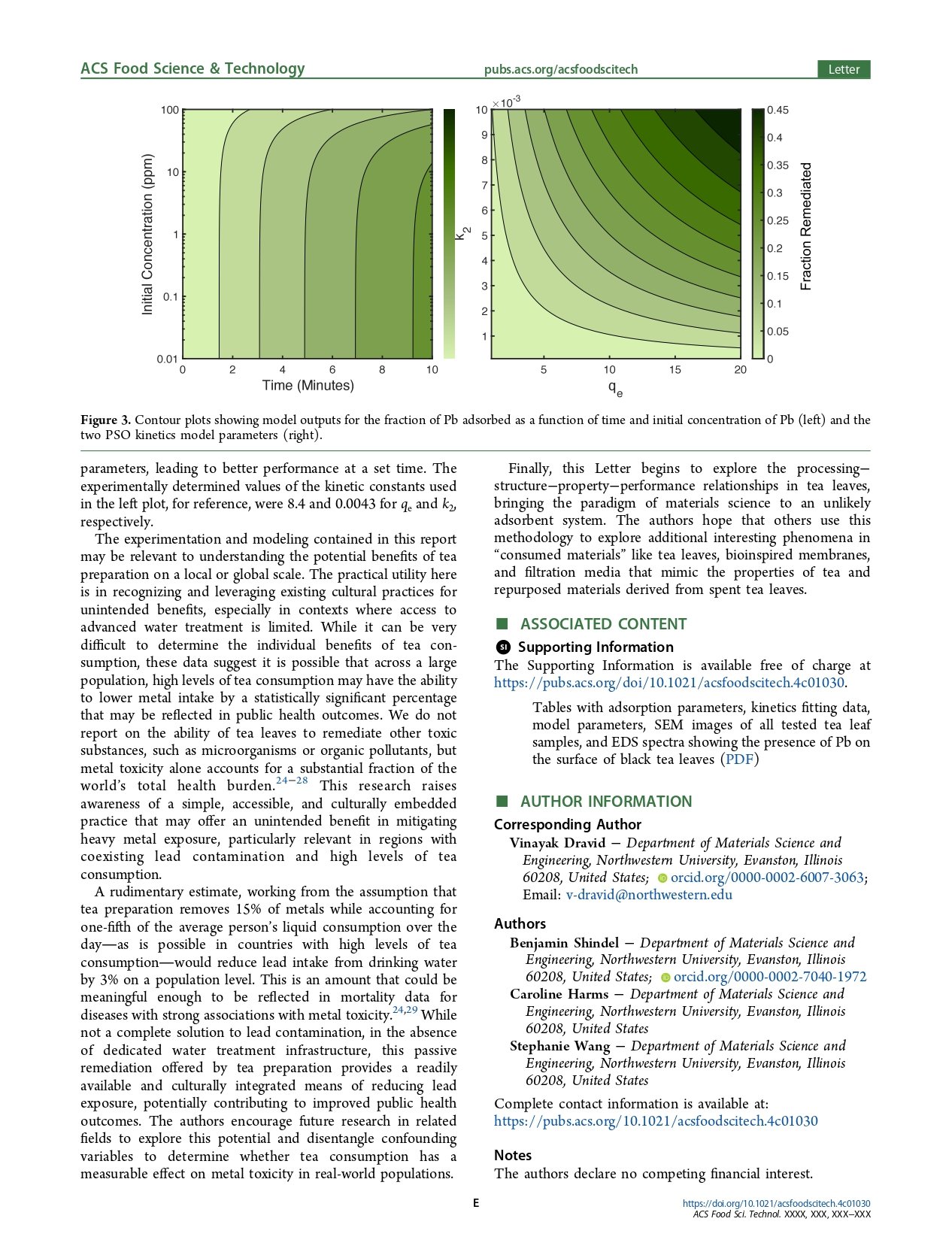
These data, presented in Figure 1b, demonstrate that the stirred room-temperature and 85 °C samples were fairly similar, with both rapidly approaching equilibrium, whereas the unstirred room-temperature sample approached thermodynamic equilibrium much more slowly. This result shows how heat impacts the kinetic performance during tea preparation but also implies that it is unlikely that heat is in and of itself affecting the thermodynamic equilibrium, as the stirred sample had similar kinetic performance. This differs from findings on the adsorption of uranium to tea leaves, which anticipated that heat may allow for greater thermodynamic capacities,14 but agrees with other findings on the adsorption of zinc to tea waste.19 Several varieties of whole tea leaves�green, black, oolong, and white�as well as two varieties of herbal teas�chamomile and rooibos�were tested in batch adsorption trials to compare their adsorptive performance. These teas represent a diverse selection of the most consumed varieties of true teas and herbal tea substitutes from around the world. Starting from an initial Pb concentration of ∼1 ppm, the final concentration of the water is shown on a log scale in Figure 1c. While most tea varieties performed quite similarly, chamomile, which has the form factor of a flower (unlike the other tea leaves tested), was an outlier in performance, remediating the water more poorly. The Yunnan black whole tea leaves that were tested in the experiments from Figure 1a−c were ground finely and tested alongside the original, whole leaves. This grinding process, which likely exposed additional surface area for adsorption, improved the performance of the tea leaves with respect to metal adsorption (shown in Figure 1d). In addition, tea bags made from cotton, cellulose, and nylon materials were tested in a fashion similar to the experiments depicted in panels c and d of Figure 1. Nylon and cotton tea bags exhibited trivial amounts of adsorption, whereas cellulose tea bags (often sold as “wood pulp” tea bags) were highly effective in remediating the metal content in the water (shown in Figure 1e). This difference in adsorption performance underscores the importance of materials selection in tea bag design, especially when additionally considering the release of microplastics associated with nylon tea bags.5 These adsorption experiments were conducted at an adsorbent loading of 100 mg in 40 mL tubes of water, agitated on a shaker table. While the focus of most of the experiments in this Letter is Pb, Cr(VI), Cu, Zn, and Cd were additionally each tested in batch adsorption trials. Pb is the heaviest of these elements by atomic weight; thus, when equivalent amounts by mass of each of these elements are found in contaminated water samples, Pb has the lowest molar concentration. The performance of black tea leaves in remediating these five elements (shown in Figure 1f) precisely follows, as expected, the trend in increasing atomic weights: Cr(VI) < Cu < Zn < Cd < Pb. The effects of pH and the use of tap water rather than deionized water for experimentation were also explored. Hydrochloric acid and sodium hydroxide were used to modify the pH of water samples before adsorption trials using black tea leaves, and the measured pH of the samples is shown alongside the remaining fraction of Pb in solution in Figure 1g. One can see that adsorption remains relatively constant across a range of pH values from 4 to 10 that encompass the likely pH values of drinking water. In Figure 1h, the results of using tap water are shown as a proof of concept. These show an adsorptive capacity comparable to that of the deionized water samples with full Langmuir isotherms tested in the experiment underlying Figure 1a. To parse the processing methods and structural features that may have an impact on adsorption properties in tea leaves, scanning electron microscopy (SEM) was performed on the six tested tea varieties. Hierarchical imaging with increasing magnification is shown for three of the samples, alongside a simplified processing flowchart, in Figure 2. White tea, which is steamed and not baked or oxidized (unlike most teas), shows a relatively smooth and unwrinkled surface. Black tea, which is wilted and fully oxidized, exhibits a more wrinkled and tortuous surface, potentially increasing the available surface area for adsorption. Researchers have evaluated the surface areas of unmodified green and black tea leaves and found them to vary between 1 and 4 m2/g.19−22 The chamomile flower, which had an adsorption capacity much lower than that of either, is smoother than black tea at the highest magnification, although it contained some moieties with complex shapes, as shown in the highest-magnification image. However, the species of chamomile flowers is different from that of true teas, which are all derived from the C. sinensis plant, and thus may have very different surface properties and internal porosities. Images of all six tea varieties can be found in Figure S2. Energy dispersive spectroscopy (EDS) was performed in a scanning electron microscope on tea samples before and after the adsorption of Pb to confirm its presence on the surface. EDS elemental mapping showing an even distribution of metal content on the surface of tea leaves can be found in Figures S3 and S4. 3.2. Modeling the Effect of Tea Preparation on Drinking Water Remediation. To interpret the data from our experimentation in a manner that provides insight into the generalized adsorptive performance during tea preparation, we created a simple mathematical model in MATLAB to calculate the predicted performance of remediating drinking water through tea preparation. The data at thermodynamic equilibrium reflected in the derived Langmuir model parameters (fitting parameters and R2 values for the Langmuir fits, as well as R2 values for linear, Freundlich, and Dubinin- Radushevksy isotherm fits performed on the data, can be found in Tables S3 and S4) were combined with parameters from the kinetics experiments. After the data from our kinetics experiments were fit with both pseudo-first-order (PFO) and pseudo-second-order (PSO) adsorption kinetics (shown in Table 1), it was found that the PSO model fit better for the heated and agitated samples (fitting parameters and R2 values can be found in Figure S1 and Tables S1 and S2), indicating that the data were in a capacity-limited regime rather than a concentration-limited regime, as well as implying that the mechanism of adsorption is likely chemisorption rather than physisorption.23 In these kinetics models, qt represents the amount of adsorbate that has been adsorbed onto the adsorbent at any given time t while qe is the equilibrium adsorption capacity. Rate constants k1 and k2 are the pseudofirst-order and pseudo-second-order rate constants, respectively. Using the ratio of adsorbent (tea) to solution (water), our model takes a first estimate of the final concentration using the Langmuir asymptote and then refines this estimate by using the whole Langmuir equation to determine the location on the Langmuir curve for a given set of conditions, and thus the adsorption capacity at that concentration at thermodynamic equilibrium. The model then factors in the fraction of the way to equilibrium reached at a set value of time, representing the amount of time for which the tea is brewed or steeped. We used this model to output a variety of contour plots, showing the fraction of metal remediated (in this case, Pb) under a combination of tea preparation conditions. These are shown in Figure 3, and parameters can be found in Table S5. The left plot shows how, at most concentrations, the fraction of metal remediated varies primarily on the basis of how long the tea is prepared. Longer brewing times intuitively correlate with improved remediation. For example, for a standard ratio of 2 g of tea leaves per cup, steeping for 5 min is estimated from our experimental data to remediate 15% of Pb ions from contaminated water samples at Pb concentrations of <10 ppm (a very highly contaminated and toxic sample of drinking water). At even higher concentrations, the adsorption capacity of the tea starts to become a limiting factor with the tea leaves “running out of room” for metal ions on their surface. The right plot shows the fraction of Pb remediated, with initial conditions of 100 ppb Pb and 5 min of brewing time, as a function of the two PSO kinetics parameters. Parameter 1 (qe), on the x-axis, represents the adsorption capacity at equilibrium, analogous to the Langmuir asymptote but at a particular concentration relevant to the kinetics experiment, rather than as the concentration approaches the high limit. Parameter 2 (k2), on the y-axis, represents the kinetic rate constant, where higher values indicate faster kinetics. This plot shows the potential effects of factors like heat, agitation, or chemical/structural processing of the tea relevant to kinetic adsorption properties on the performance during tea preparation expressed as the fraction of Pb remediated. Higher heat during adsorption or tea leaves processed to improve porosity or surface area may drive up the values of the two parameters, leading to better performance at a set time. The experimentally determined values of the kinetic constants used in the left plot, for reference, were 8.4 and 0.0043 for qe and k2, respectively. The experimentation and modeling contained in this report may be relevant to understanding the potential benefits of tea preparation on a local or global scale.
and the last part
The experimentation and modeling contained in this report may be relevant to understanding the potential benefits of tea preparation on a local or global scale. The practical utility here is in recognizing and leveraging existing cultural practices for unintended benefits, especially in contexts where access to advanced water treatment is limited. While it can be very difficult to determine the individual benefits of tea consumption, these data suggest it is possible that across a large population, high levels of tea consumption may have the ability to lower metal intake by a statistically significant percentage that may be reflected in public health outcomes. We do not report on the ability of tea leaves to remediate other toxic substances, such as microorganisms or organic pollutants, but metal toxicity alone accounts for a substantial fraction of the world’s total health burden.24−28 This research raises awareness of a simple, accessible, and culturally embedded practice that may offer an unintended benefit in mitigating heavy metal exposure, particularly relevant in regions with coexisting lead contamination and high levels of tea consumption. A rudimentary estimate, working from the assumption that tea preparation removes 15% of metals while accounting for one-fifth of the average person’s liquid consumption over the day�as is possible in countries with high levels of tea consumption�would reduce lead intake from drinking water by 3% on a population level. This is an amount that could be meaningful enough to be reflected in mortality data for diseases with strong associations with metal toxicity.24,29 While not a complete solution to lead contamination, in the absence of dedicated water treatment infrastructure, this passive remediation offered by tea preparation provides a readily available and culturally integrated means of reducing lead exposure, potentially contributing to improved public health outcomes. The authors encourage future research in related fields to explore this potential and disentangle confounding variables to determine whether tea consumption has a measurable effect on metal toxicity in real-world populations. Finally, this Letter begins to explore the processing−structure−property−performance relationships in tea leaves, bringing the paradigm of materials science to an unlikely adsorbent system. The authors hope that others use this methodology to explore additional interesting phenomena in “consumed materials” like tea leaves, bioinspired membranes, and filtration media that mimic the properties of tea and repurposed materials derived from spent tea leaves.
As always a single study means nothing. You have to wait for the meta study that collects all the other studies and determines the general trend
Didn’t i just read an article on teabags leeching microplastics?
There is such thing as loose leaf tea where you don’t use tea bags when brewing.
After a bit further reading of the article it looks like they also found that certain types of tea bags helped.
Specific type of tea bag that’s made out of plastic most of the ones you find are not of the same material. It’s the plastic tea bags that are causing it.
Even cellulose and paper bags were tested and had plastics in the glues and binders in the material. Unfortunately you need to look for manufacturers that explicitly say they’re plastic-free or buy loose leaf. I have a bunch of bagged tea I bought before I knew, and I’ve been ripping open the bags and dumping the tea in my infuser.
So… wait. Did I read this right?
No matter how they brewed it, they filtered it a second time through cellulose. While steep time seems to be a factor, isn’t the cellulose doing the heavy lifting here?
Tannins and other compounds in tea can bind to heavy metals. Tho’ really I’d suggest not drinking water that has heavy metals in it, if at all possible. Edit: And for that matter, it would be amazing to avoid heavy metals in food as well.
Yeah, hopefully there was a control done with just water (no tea) at the same temperature also passed through the second filter. I can’t seem to get the whole paper without a subscription (or school/work/library account). Anyone have access and can confirm the details?
there was
Is it the same for looseleaf or bagged?
cellulose bags do some absorbing too:
The cellulose tea bag was found to have a higher binding affinity but a lower asymptotic limit compared with those of the tea itself. The different tea leaves had similar values for both Langmuir parameters, with the Lipton tea─which was finely ground, packaged tea─having slightly increased properties compared to those of the whole tea leaves. The higher values for the Lipton tea line up with subsequent experimentation on the effect of fineness
The team found that cellulose tea bags work the best at adsorbing toxic metals from the water while cotton and nylon tea bags barely adsorbed any contaminants at all—and nylon bags also release contaminating microplastics to boot.
Tea type and the grind level also played a part in adsorbing toxic metals, with finely ground black tea leaves performing the best on that score.
But the most significant factor was steeping time: the longer the steeping time, the more toxic metals were adsorbed.
I’m glad I over- steep my tea, also started using loose leaf due to the microplastics found in many grocery store selections
Uhh, which brands use nylon tea bags please?









